Promising Multiple Sclerosis Treatment Fails Testing
The difficult balance between incentivizing medical invention while controlling costs
I’m very sorry for my absence. My son and I fell very sick with a stomach bug and we are finally back rejoining the world. That was rough. Thanks for your patience.
Whenever I’m sick I am reminded of the quote: “A healthy person wants a million things. A sick person wants just one.” This made me want to tell the story of the medical therapy ATA188.
A month ago I shared the story of an incredible invention that will cure the terrible pain from Sickle Cell Disease. Miraculously, there are now two drugs approved by the FDA that completely cure this disease. It is truly incredible.
But we also discussed that the drugs Casgevy and Lyfgenia are priced at $2 million to $3 million per treatment!
A shocking price tag to say the least.
It’s understandable why someone might look at that price tag and get angry with the company that is selling the products. How dare they charge millions of dollars for something that is supposed to help cure people?
This brings up a big hairy question: How much should medical treatments cost? How should they be priced?
In the case of these two drugs, the alternative treatment for the patients costs $4 million over a lifetime and doesn’t provide relief from pain. So even at $2-$3million, the new treatment is less expensive, better for the patient, and saves the patient from a lot of ongoing pain.
But even so, I can totally understand why someone would still look at the $3 Million price tag and think that the pharmaceutical company that is selling the drug is a money hungry monster. I get it.
But not all drugs that are tested are successful like Casgevy and Lyfgenia.
Take for instance, ATA188 from Atara Biotherapeutics.
ATA188 is an immune therapy designed to treat multiple sclerosis (MS). This therapy is working on the newly developed theory that MS might be caused by the Epstein-Barr virus (EBV) in someway. This really cool study of over 10,000,000 military personnel showed a strong correlation between EBV and MS.
ATA188 hoped to use healthy t-cells to target EBV cells to reduce or halt the progression of MS, offering a new approach to treating this chronic disease.
I have two close family members that suffer with MS so I have been following this treatment fairly closely in hopes that it could offer some answers and potentially a solution for my loved one.
A little history on drug/therapy development:
Medical treatments like drugs and immunotherapies like ATA188 usually involve many partners and organizations that work together to test and develop these medical inventions. For ATA188, Memorial Sloan-Kettering Cancer Center and QIMR Berghofer Medical Research Institute were involved. Atara Biotherapeutics was the company that was commercializing the technology.
What does this mean? Well basically the universities are often the ones that invent the new therapy or drug but they aren’t that great at testing, getting FDA approval, and bringing the drug/therapy to market so that patients can buy the product to help them.
So usually the Universities partner with a company or a bunch of investors to help get the innovation from the lab into the real world. That’s where a company like Atara Biotherapeutics comes in.
Atara enters into a licensing deal with the University and the people that invented ATA188 where, in most cases, the University/Inventor give the company a license to commercialize the invention, and in exchange they get fees and royalties if the product is sold to customers and patients.
But, the catch for Atara Biotherapeutics is that now they must pay for the testing and trials that go into getting the drug/therapy approved. This can take a very long time and cost a hell of a lot of money.
For instance, the phase 1 trial for ATA188 was way back in 2017. As you may recall, in most cases there are 3 phases of trials. I’m greatly simplifying this but in general here is what the trials do:
Phase 1: Get a small group of people, give them the drug/treatment, make sure it’s safe and test what dosage to give.
Phase 2: Get a larger group of people and make sure it’s safe and figure out what the best dosage to give is.
Phase 3: Get an even larger group to test that the drug is safe and evaluate how it compares to the alternative treatments on the market and placebo.
All of us in the MS community were thrilled when we saw the results of the Phase 1 trial. 9 of the 24 people in the trial had improvement in their disability! This got a lot of us very excited that we may finally have some relief for MS patients.
Of course, this early trial data got Atara Biotherapeutics excited too! With this result they wanted to put more money into funding Phase 2 trials.
Here is how they designed the Phase 2 study:
“The Phase 2 EMBOLD study for ATA188 is a randomized, double-blind, placebo-controlled dose-expansion trial designed to evaluate the efficacy and safety of ATA188 in patients with progressive multiple sclerosis (PMS). The study, which began with the enrollment of the first patient in June 2020, aimed to measure changes in disability measures compared to baseline, particularly sustained disability improvement (SDI) over time. Additionally, the study included multiple measures of patients' function as well as various biomarkers.”
So to recap the timeline, the phase 1 study started in 2017 and the phase 2 portion of the study didn’t start until mid 2020.
This is one of the things that contributes to the cost of drugs. It just takes a long time to test them and make sure they are safe. Not only did it take 3 years from phase 1 to phase 2, but phase two itself was designed to last 2 years.
Sadly, in November of 2023, we got the first results from the Phase 2 trials and they were bad. The therapy had failed.
Here is the technical jargon:
“ATA188 did not meet its primary endpoint of change in confirmed disability improvement among patients with non-active progressive multiple sclerosis after 12 months of treatment. A 6% disability improvement was observed in the ATA188 group at the 12-month findings, which was significantly lower than the 33% observed in the Phase 1 study. The expected rate of confirmed disability improvement for patients on placebo was 16% at 12 months, exceeding the 4% to 6% expected rate.”
Basically, after 6 years of testing and a lot of hope and promise, the people that received the treatment in the study did WORSE than those that received a placebo. Somehow 16% of the people that received the placebo got better but only 6% of the people that received the treatment.
How could that be? How could people that didn’t get any treatment (placebo) do better than those that got the treatment? — We don’t know. But this is exactly why we do these tests. Not only do we have to make sure they are safe, but we also have to make sure that they perform better than a placebo.
So what happens with ATA188? It’s not certain yet. Atara Biotherapeutics has fired 25% of their staff including a VP and their Chief Medical Officer. The severance payments to those employees alone, just for firing them, was more than $4million.
So how much did Atara Biotherapeutics spend on ATA188?
It’s not easy to tell exactly how much was spent on ATA188, but what we do know is that Atara spent a lot on research and development:
In 2020, the total R&D expenses were $244.65 million.
In 2021, the total increased slightly to $282.001 million.
In 2022, the total slightly decreased to $272.533 million
Now of course, not all of that money was spent on ATA188 and these are just 2020, 2021 and 2022 numbers. So lets be very conservative, let’s say that $150,000,000 was spent to test ATA188. I think that is a very conservative number, but I can’t be sure.
That money is now gone. $150,000,000 out the door, never to return.
Not only is the money gone, but several medical professionals spent their time and effort for almost 7 years working on the testing for this drug. That time and effort is all gone now.
Where did the $150 million come from?
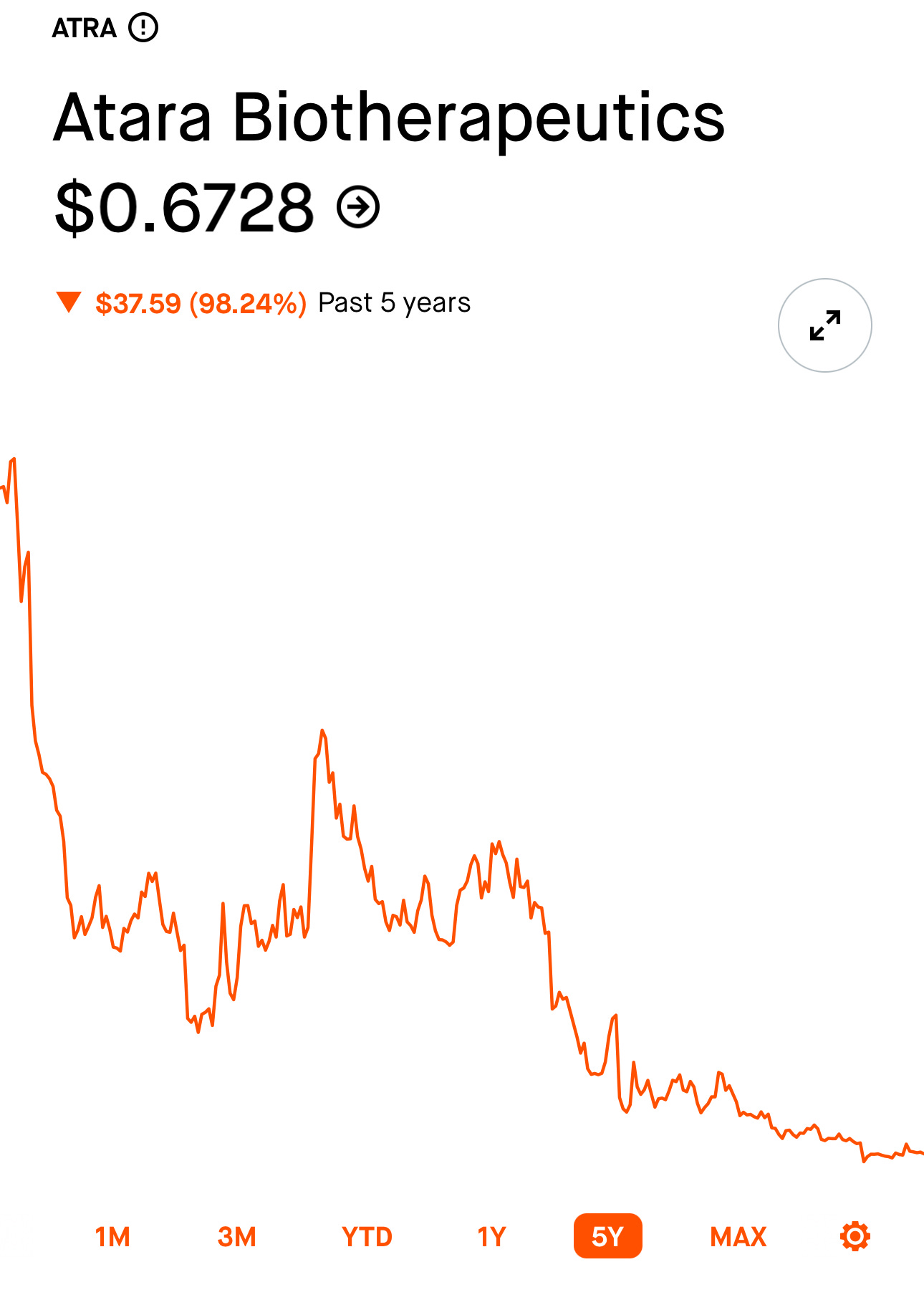
Atara is a publicly traded company meaning that anyone of the public can purchase shares in the company. The owners of the company are the ones that lost the $150,000,000. Take a look at their stock price:
Anyone that invested in Atara for the past 5 years, most likely lost a lot of money. So why did they invest? They invested because they believed ATA188 would work. Maybe they were suffering with MS so they wanted to put their money into a company that might provide a cure. Maybe they were just money seeking investors that read the Phase 1 study, got excited, and thought they could invest in the next Novo Nordisk stock that was going to the moon!
Whatever their rational, the investors that put money into Atara were the ones that took the financial risk to test ATA188. They risked their own capital to see if this treatment could help other humans.
On this bet, they lost. This drug failed and it probably cost them about $150,000,000! An incredible amount of money. Hard to imagine really.
It’s estimated that only 1 in 5,000 drugs/therapies that are tested make it to human clinical trials. And of the drugs / therapies that make it to human clinical trials, only 1 in 10 will ever get regulatory approval. That’s about a 0.002% chance that a therapy may be approved!
So why would anyone take the risk with their own money when there is only a 0.002% chance of success?
It’s easy to answer: They invest for the chance to make a lot of money.
Think about it like this…
Let’s say you are in vegas and you walk past a slot machine that says there is a .002% chance of winning. If you put in $1 you have a .002% chance of winning $10. Would you do it? Probably not.
But what about $1 bet for a .002% chance to win $1,000? Well now it’s a bit more exciting.
What about $1 bet for a .002% chance at winning $1,000,000,000? That’s a great bet to take!
As much as we like to think of medical inventions as being altruistic inventions that are solely accomplished by the brilliant scientist in a lab - that’s not how it actually works.
The drug and therapy discovery and invention process is a beautiful dance between several parties. The universities and government providing foundational support and investment, the government evaluating the safety and effectiveness of the treatments, business people establishing companies to commercialize these inventions, and investors providing the financial support to fund the testing.
So how does this all relate to the $2-$3 million Sickle Cell Therapy treatments?
Thanks for sticking with me as I got to the point. While I can see how someone would look at a $3million treatment and be shocked and outraged - we need to keep in mind how many ATA188’s are out there. How many drugs were tested that never saw the light of day? How much money was spent on developing drugs that didn’t get approved?
We must keep that in mind when thinking of healthcare costs and drug costs. There is a delicate balance that needs to be struck for a healthy society. We don’t want our health care costs to run out of control and eat up our economy. But we also want to make sure that we keep incentivizing new invention and drug discovery.
In order to do this we need:
scientists that are incentivized financially to keep inventing new treatments
investors that are incentivized financially to keep investing in new treatments.
I firmly believe that we need both of these things in order to maximize our society’s ability to invent new medical breakthroughs. As much as we like to hope that our medical inventions are done by noble scientists that don’t care about money - that doesn’t seem to be the reality.
Some people argue that we need to remove the “profit motive” from healthcare in order to serve patients better. I can sympathize with this view because it makes emotional sense. You don’t, for instance, want someone deciding who lives or dies based on how much money they have. I totally understand that argument.
But when it comes to medical invention and discovery, the profit motive is a very important component in my mind. To me personally, I think the investors that risked $150,000,000 of their own money with the hopes that Atara would invent a cure for MS are heroes! They risked a lot of their own capital in hopes that it would help other humans. It didn’t work out this time and they lost all or most of their money.
But if that investment had worked out, for me personally, I would hope that every single investor in ATA188 would be rewarded for their risk by getting filthy rich. Not because they are “profiting off the sick” but because they were the ones that invested in making the sick healthy. And to me, that’s the kind of investments we should be rewarding as a society.



Glad you two are feeling better. Missed you!
Love this framing "Not because they are “profiting off the sick” but because they were the ones that invested in making the sick healthy."